InnAVasc Medical, Inc.
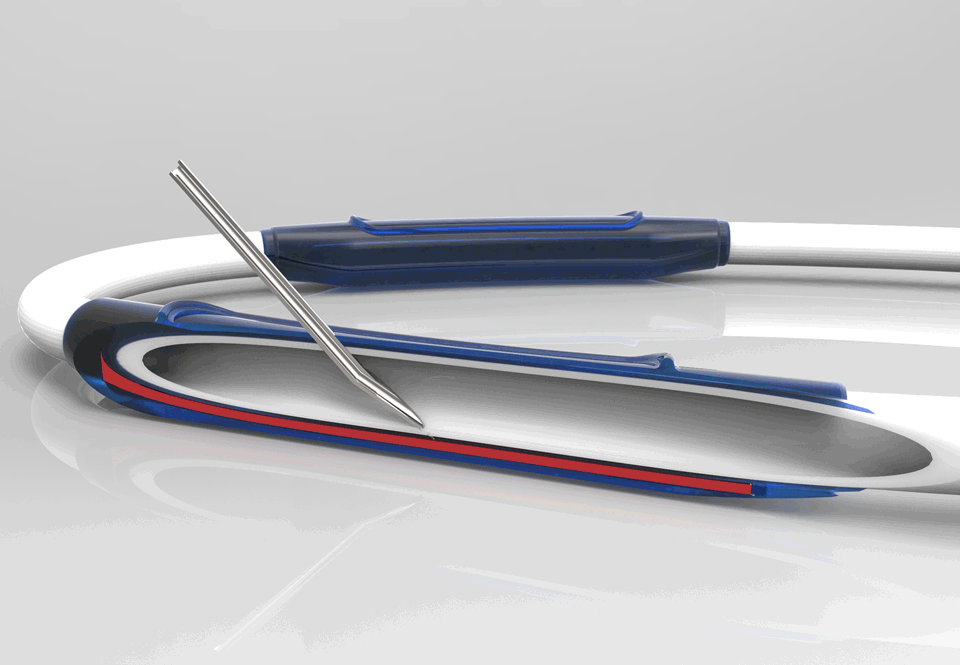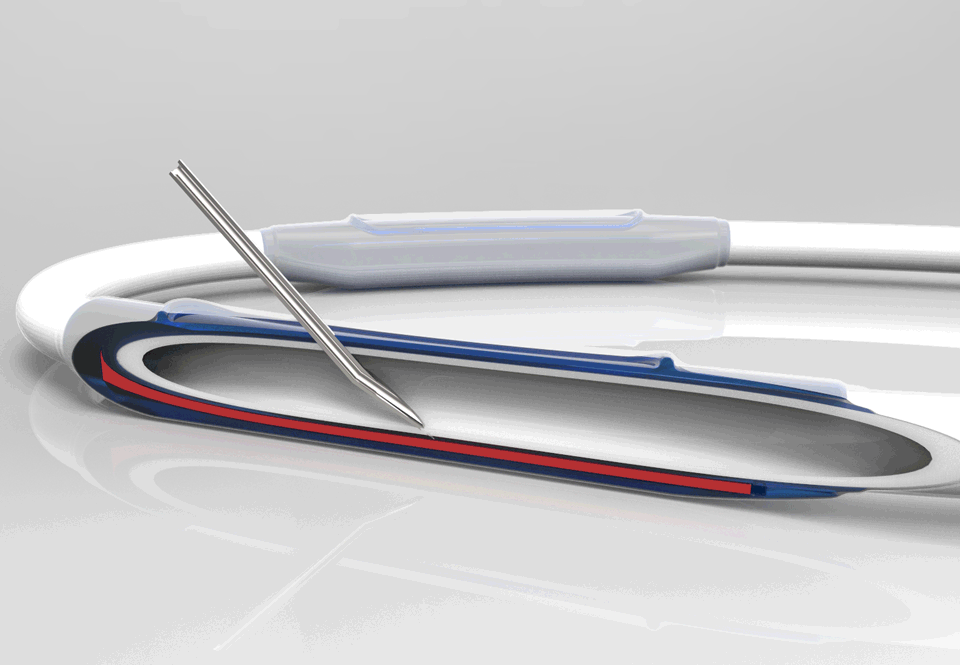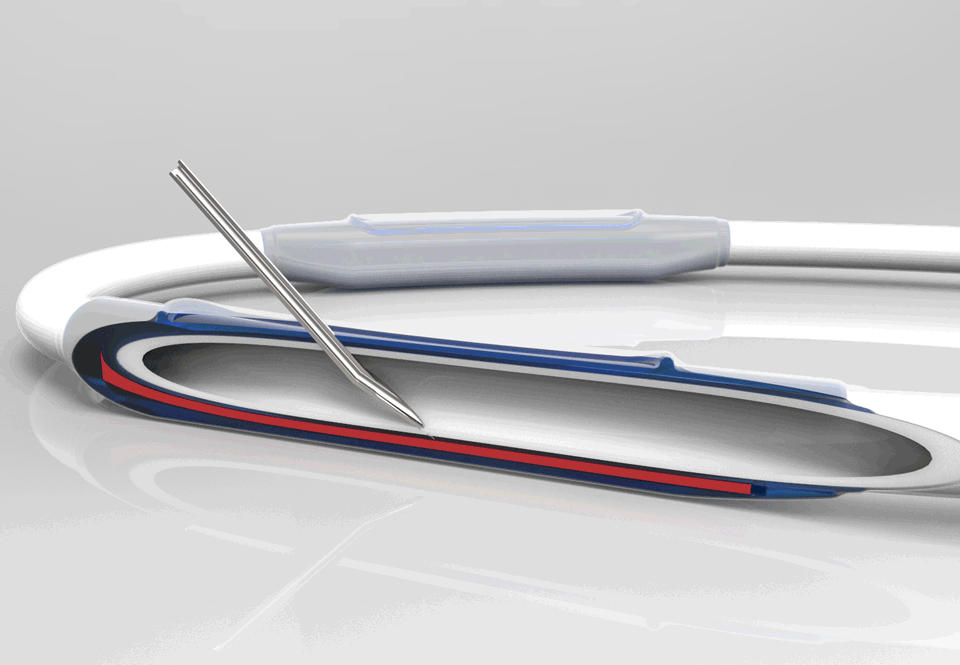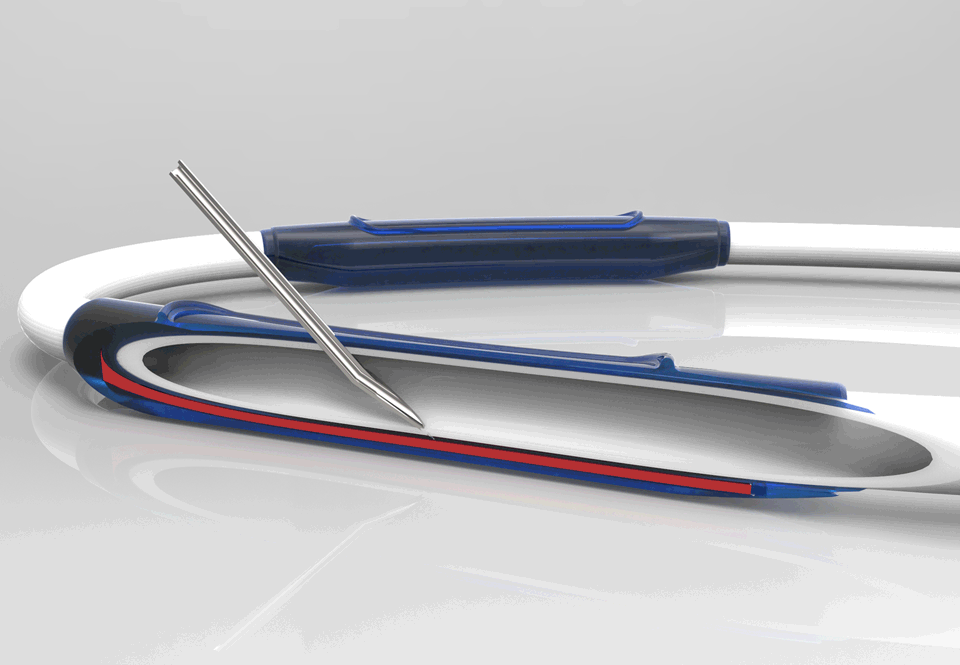
InnAVasc Medical, Inc. is a medical device company founded by Duke University surgeons and scientists, which designs and develops products for vascular access for hemodialysis. The Company’s first technology is an arteriovenous graft (AVG) modification that incorporates two multilayer cannulation chambers, with low bleed technology, that are resistant to posterior and sidewall needle penetration and injury: the Bullet Proof Vascular Access Graft.
This production method modifies existing synthetic vascular grafts for immediate hemodialysis access, prevents needle cannulation injuries, and facilitates graft access and safety.
Milestones
- IP priority March 16, 2011
- 1st Pre-clinical studies and proof of concept completed February 10, 2012
- InnAVasc established May 13, 2013
- Initial round of investment funding October 2014
- European patent granted December 2014
- FDA Q-Submission meeting December 2014 - confirms 510(k) pathway
- Established manufacturing partner for device finalization January 2015
- Duke University DTMI Collaborative Pilot Grant Awarded April 2015 - $100K award
Grants And Awards
DUKE UNIVERSITY DTMI COLLABORATIVE PILOT GRANT AWARDED APRIL 2015
$100K AWARD
DTMI GRANT ARTICLE
$100K award - Learn More
CHARING CROSS 2015 INNOVATION SHOWCASE
Winner of “Speed Dating” and “Dragons Den” sessions
vascularnews.com